I believe this Tsunami Debris is directly in the path of migrating whales to Alaska…
From BBC
http://www.bbc.co.uk/news/world-us-canada-18349741
 Tapping into the Ocean
Tapping into the Ocean
By Nikolay Voutchkov
Seawater desalination produces fresh, low-salinity potable water from seawater via membrane separation or evaporation. The mineral/salt content of the water is usually measured by the water quality parameter total dissolved solids (TDS), in milligrams per liter (mg/L) or parts per thousand (ppt).
The World Health Organization and the U.S. EPA, under the Safe Drinking Water Act, have established a maximum TDS concentration of 500 mg/L as a potable water standard. This TDS level can be used as a classification limit to define potable water.
Typically, water of TDS concentration higher than 500 mg/L and low or equal to 15,000 mg/L is classified as brackish. Natural water sources such as sea, bay or ocean waters usually have TDS concentration higher than 15,000 mg/L and are generally classified as seawater.
For example, Pacific Ocean seawater along the U.S. West Coast has a TDS concentration of 33,500 mg/L of which approximately 75% is sodium chloride.
Approximately 97.5% of the water on the planet is located in the oceans and therefore is classified as seawater. Of the 2.5% of the planet’s fresh water, approximately 70% is in the form of polar ice and snow, and 30% is groundwater, river and lake water, and air moisture. Even though the volume of the earth’s water is vast, less than 10 million of the 1.4 billion cubic meters of the water on the planet are of low salinity and suitable for use after applying conventional water treatment only. Desalination provides means for tapping the world’s main water resource—the ocean.
Measuring salinity
Most meters measure the electrical conductivity of water.

Batteries are connected to two wires (electrodes). Salty water will conduct electricity between the two electrodes, the saltier the water, the more electricity is conducted.
The unit of electrical measurement used is Siemens. The meter we use in the field measures milliSiemens (mS) per centimetre (the electrodes are one centimetre apart).
Often the literature will use microSiemens (uS) and EC standing for electrical conductivity. These two units are the same. To convert milliSiemens to EC’s or uS multiply the reading by 1000. The meter in the diagram shows 1.65 mS which is 1650 uS or EC’s.
Water Salinity Levels for Use
Source/Use uS/cm or EC Source uS/cm or EC
distilled water 0 limit poultry 5 800
Murray R. (mouth) 790 limit beef cattle 16 600
desirable limit people 830 limit adult sheep 23 000
absolute limit people 2 500 Pacific Ocean 58 000
Conversions
deciSiemens per meter (dS/m) = milliSiemens per cm (mS/cm) times by 1000 equals
microSiemens per cm (uS/cm) = Electrical Conductivity units (EC’s)
Example 1.5 dS/m = 1.5 mS/cm = 1500 uS/cm = 1500 EC
Graphics are different resolutions and FREE license
http://en.wikipedia.org/wiki/File:WOA05_sea-surf_SAL_AYool.png
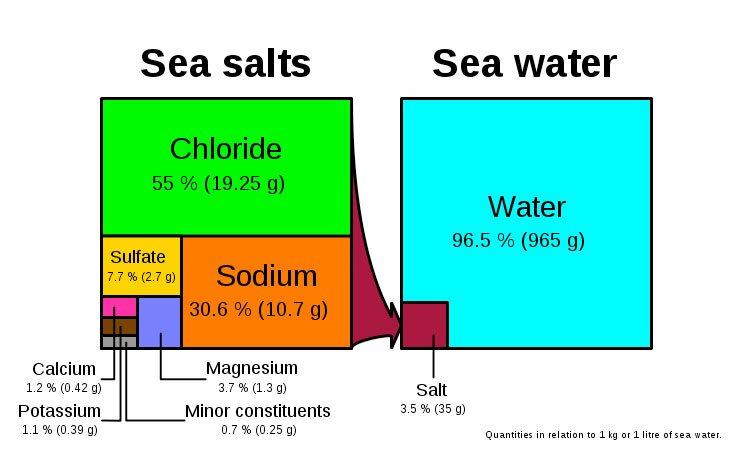
Diagram showing concentrations of various salt ions in seawater: Cl− 55%, Na+ 30.6%, SO2−
4 7.7%, Mg2+ 3.7%, Ca2+ 1.2%, K+ 1.1%, Other 0.7%. Note that the diagram is only correct in units of wt/wt, not wt/vol or vol/vol.
I found this a good definition of a Marine Biologist. But, it is one of many…
http://www.wisegeek.com/what-is-a-marine-biologist.htm
A marine biologist is usually a person with advanced degrees in life sciences. He or she will study life forms of the ocean from a scientific perspective, and may hold specific bachelors, masters, or PhDs in biology, marine biology, and/or chemistry.
There are many different areas in which a marine biologist may work. Very few marine biologists work studying ocean dwelling mammals like whales or dolphins. Yet people often think this is the only area in which a marine biologist can work.
There are some good data presentations here:
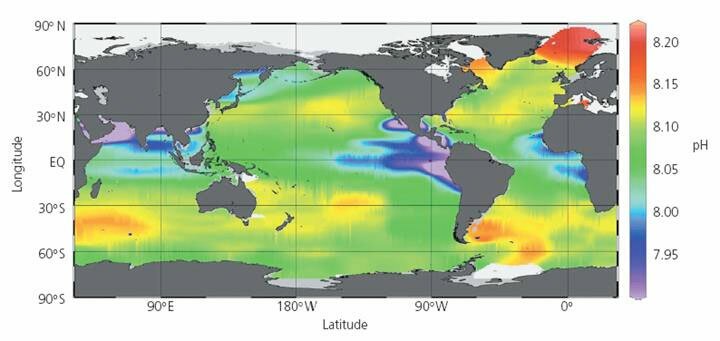
http://www.appinsys.com/globalwarming/OceanAcidification.htm
A student definition???
Definition: Acidic: containing or having the properties of an acid, “Pertaining to substances that have a pH lower than 7” [http://www.biology-online.org/dictionary/Acidic]. While the term acidification refers to decreasing pH, the term acidic is only valid when the pH is less than 7.
The concern is that as the atmospheric CO2 increases, it increases the CO2 content of the ocean, resulting in increased carbonic acid (H2CO3). The following equation shows the basic equilibrium equation involved in the water (with <=> denoting equilibrium):
CO2 + H2O <=> H2CO3 <=> H+ + HCO3-